Do you seek for 'chemistry ia electrolysis of metal sulphate solutions essay'? You can find all the information on this website.
Table of contents
- Chemistry ia electrolysis of metal sulphate solutions essay in 2021
- Electrolysis of water chemistry ia
- Chemistry ia examples
- Enzyme chemistry ia
- Chemistry ia ideas
- Personal engagement chemistry ia example
- Ib chemistry ia lab report example
- Electroplating chemistry ia
Chemistry ia electrolysis of metal sulphate solutions essay in 2021
 This image shows chemistry ia electrolysis of metal sulphate solutions essay.
This image shows chemistry ia electrolysis of metal sulphate solutions essay.
Electrolysis of water chemistry ia
 This picture demonstrates Electrolysis of water chemistry ia.
This picture demonstrates Electrolysis of water chemistry ia.
Chemistry ia examples
 This picture illustrates Chemistry ia examples.
This picture illustrates Chemistry ia examples.
Enzyme chemistry ia
 This image illustrates Enzyme chemistry ia.
This image illustrates Enzyme chemistry ia.
Chemistry ia ideas
 This image representes Chemistry ia ideas.
This image representes Chemistry ia ideas.
Personal engagement chemistry ia example
 This picture representes Personal engagement chemistry ia example.
This picture representes Personal engagement chemistry ia example.
Ib chemistry ia lab report example
 This image representes Ib chemistry ia lab report example.
This image representes Ib chemistry ia lab report example.
Electroplating chemistry ia
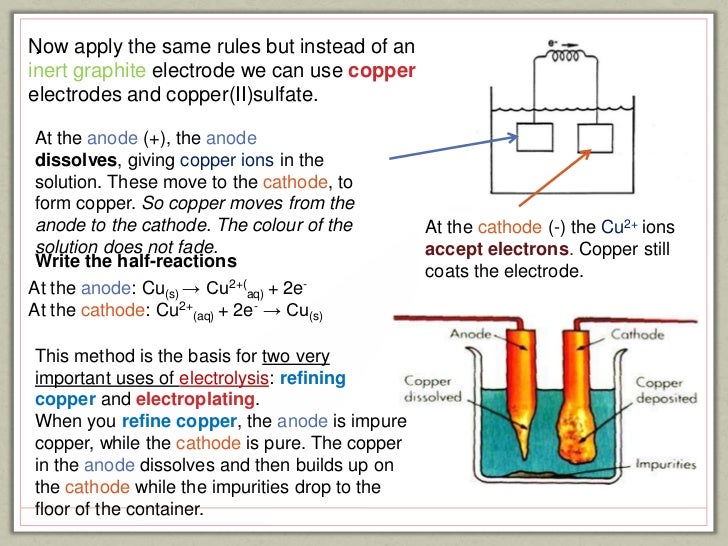 This picture shows Electroplating chemistry ia.
This picture shows Electroplating chemistry ia.
How is the mass of an element affected during electrolysis?
“The mass of any element deposited during electrolysis is directly proportional to the number of coulombs of electricity passed” From this statement we can say that the amount of electricity that we pass in the circuit will directly affect the rate of electrolysis.
How does distance between electrodes affect the rate of electrolysis?
Separation of electrodes – Decreased distance between the electrodes increases the rate of electrolysis Quantity of copper sulphate solution- the amount of copper sulphate should remain constant to make it a fair test. Thermometer (± 0.05 ËšC) The same concentration of the solution will be used
How is the mass of nickel measured after electrolysis?
The mass of nickel deposited at the cathode after electrolysis will be measured for results. This will be determined by weighing the nickel electrodes before the experiment and after electrolysis. For this, a electronic balance will be used to weigh them.
How does the rate of electrolysis of copper sulphate increase?
Electrolysis Of Copper Sulphate Chemistry Essay. My hypothesis based on faraday’s first law of electrolysis is that as current increases and more amps are passed through the circuit the rate of electrolysis will increase. The rate of electrolysis can be seen from the increase in mass of cathode.
Last Update: Oct 2021