Do you want to find 'synthesis of asa'? Here, you will find all the stuff.
Deductive reasoning and Characterization of Aspirin Name Chemical formula Molecular Weight (g/mol) Melting Point (° C) Boiling Compass point (° C) Salicylic Acid C7H6O3 211 Acetic Anhydride C4H6O3 Acetic Acid C2H4O2 Phosphoric Acid H3PO4 158 Sep 28 2021
Table of contents
- Synthesis of asa in 2021
- Preparation of aspirin class 12
- Synthesis of aspirin reaction mechanism
- Synthesis of aspirin balanced equation
- Synthesis of aspirin lab
- Synthesis of acetylsalicylic acid lab report
- Synthesis of aspirin book
- Synthesis of aspirin mechanism
Synthesis of asa in 2021
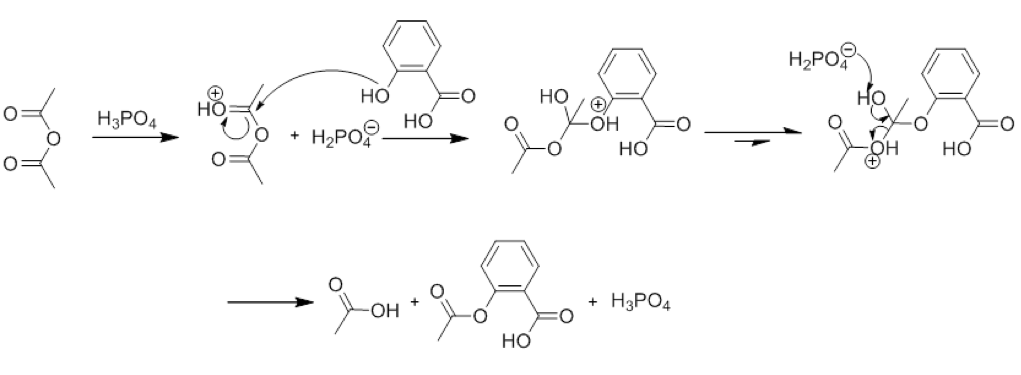 This image illustrates synthesis of asa.
This image illustrates synthesis of asa.
Preparation of aspirin class 12
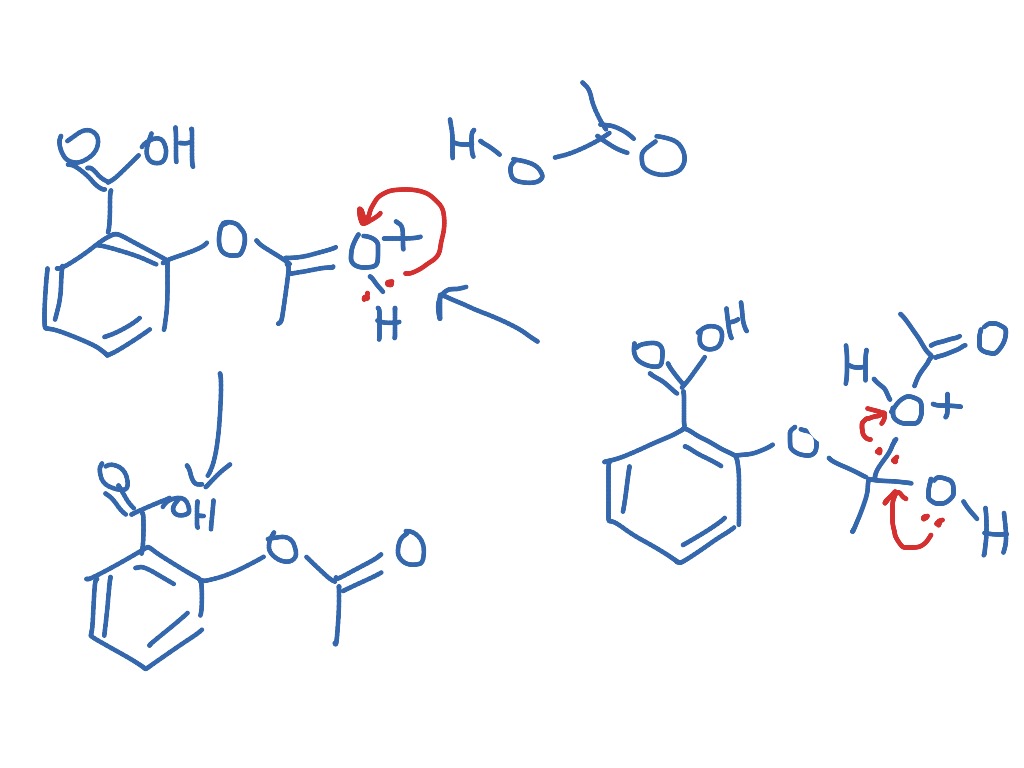 This picture shows Preparation of aspirin class 12.
This picture shows Preparation of aspirin class 12.
Synthesis of aspirin reaction mechanism
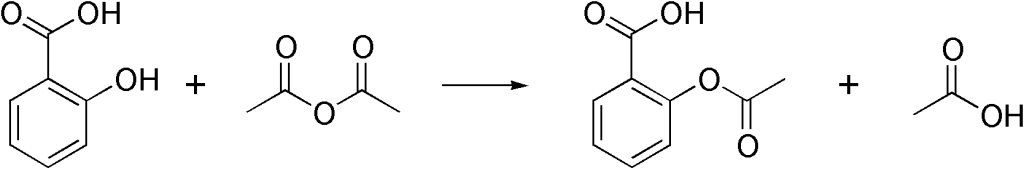 This picture demonstrates Synthesis of aspirin reaction mechanism.
This picture demonstrates Synthesis of aspirin reaction mechanism.
Synthesis of aspirin balanced equation
 This picture demonstrates Synthesis of aspirin balanced equation.
This picture demonstrates Synthesis of aspirin balanced equation.
Synthesis of aspirin lab
 This image representes Synthesis of aspirin lab.
This image representes Synthesis of aspirin lab.
Synthesis of acetylsalicylic acid lab report
 This image illustrates Synthesis of acetylsalicylic acid lab report.
This image illustrates Synthesis of acetylsalicylic acid lab report.
Synthesis of aspirin book
 This picture representes Synthesis of aspirin book.
This picture representes Synthesis of aspirin book.
Synthesis of aspirin mechanism
 This image demonstrates Synthesis of aspirin mechanism.
This image demonstrates Synthesis of aspirin mechanism.
How is the synthesis of aspirin and acetic acid characterized?
Synthesis and Characterization of Aspirin. Reaction of Salicylic Acid and Acetic Anhydride to form Aspirin and Acetic Acid The rings shown in the Salicylic acid and Aspirin molecules are hexagonal rings of carbon compounds, with alternating single and double bonds as indicated by the double lines.
Who was the first to synthesise acetylsalicylic acid?
When Bayer applied for a patent, it turned out that acetylsalicylic acid had been synthesized earlier, even though it was probably not as pure as Hoffmann’s.
Why is vacuum filtration used in synthesis of aspirin?
Vacuum filtration can be used to help dry out a sample as well. Another laboratory technique used in this experiment is recrystallization. This process is necessary because Aspirin is crystalline at room temperature, but when it is synthesized, it is in solution at a higher temperature.
What are the steps in the recrystallization of aspirin?
Synopsis. The main procedures are preparation of aspirin, recrystallisation of aspirin and lastly determining the melting point of the aspirin. For preparation of Aspirin, acetic anhydride is added to the measured amount of salicylic acid. Sulphuric acid is added and heated for a short period to complete reaction.
Last Update: Oct 2021